in focus
PAPER PUBLISHED: Zaijun ET AL., eLIFE 2018
May 29th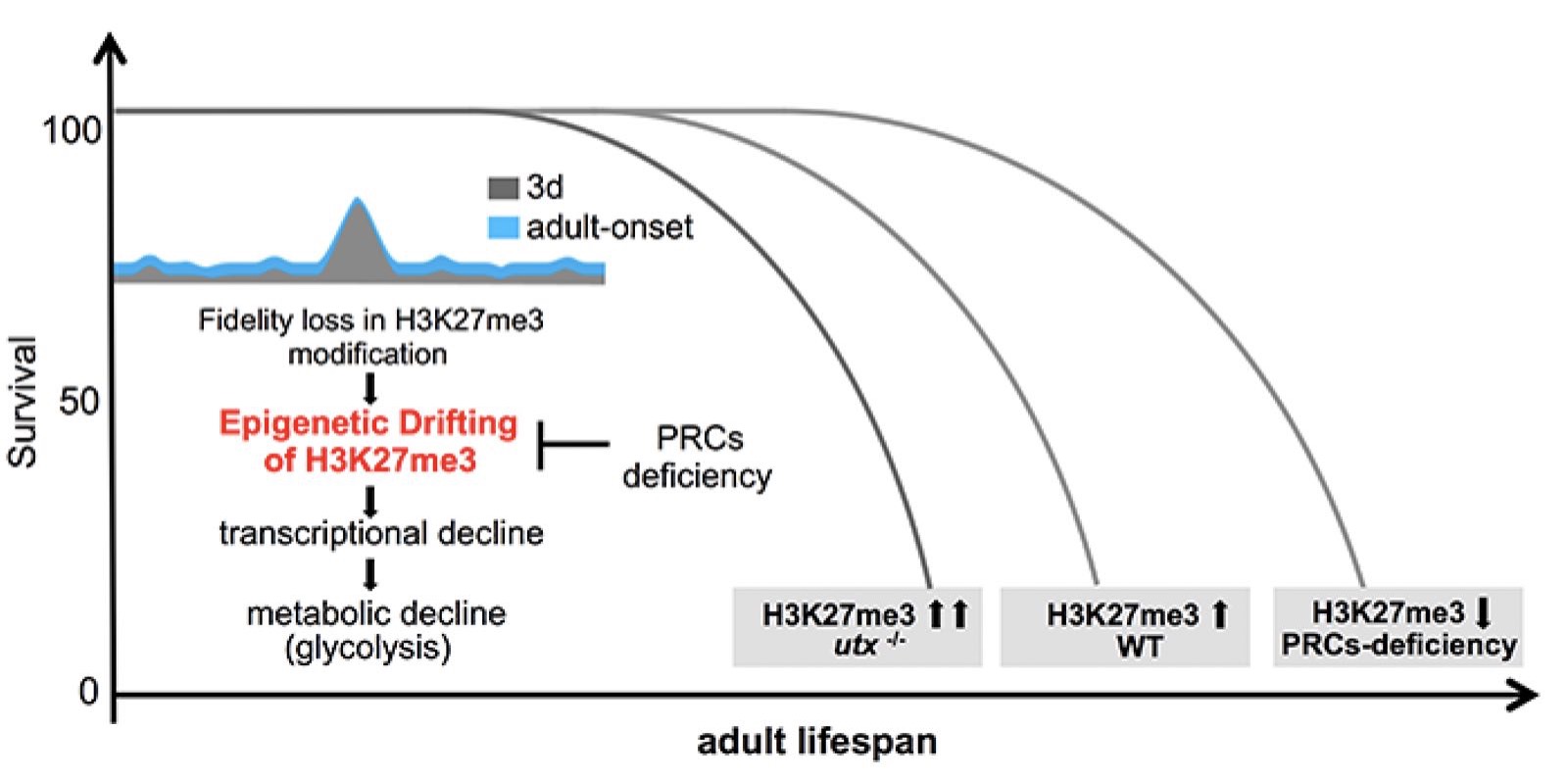
Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila
Epigenetic alteration has been implicated in aging. However, the mechanism by which epigenetic change impacts aging remains to be understood. H3K27me3, a highly conserved histone modification signifying transcriptional repression, is marked and maintained by Polycomb Repressive Complexes (PRCs). Here, we explore the mechanism by which age-modulated increase of H3K27me3 impacts adult lifespan. Using Drosophila, we reveal that aging leads to loss of fidelity in epigenetic marking and drift of H3K27me3 and consequential reduction in the expression of glycolytic genes with negative effects on energy production and redox state. We show that a reduction of H3K27me3 by PRCs-deficiency promotes glycolysis and healthy lifespan. While perturbing glycolysis diminishes the pro-lifespan benefits mediated by PRCs-deficiency, transgenic increase of glycolytic genes in wild-type animals extends longevity. Together, we propose that epigenetic drift of H3K27me3 is one of the molecular mechanisms that contribute to aging and that stimulation of glycolysis promotes metabolic health and longevity.
Read more
PAPER PUBLISHED: HUI ET AL., DEVELOPMENT 2016
February 1st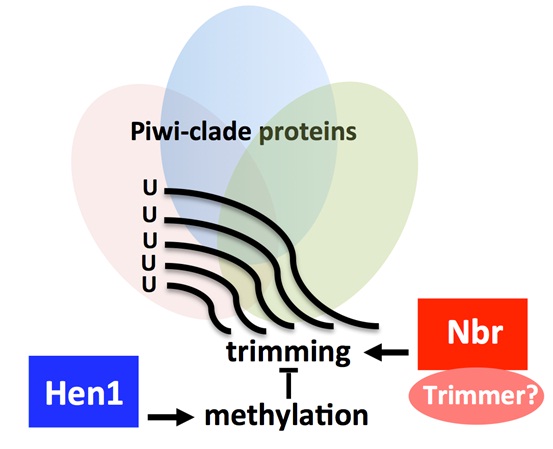
Antagonistic roles of Nibbler and Hen1 in modulating piRNA 3′ ends in Drosophila
In eukaryotes, aberrant expression of transposable elements (TEs) is detrimental to the host genome. Piwi-interacting RNAs (piRNAs) of ∼23 to 30 nucleotides bound to PIWI clade Argonaute proteins silence transposons in a manner that is strictly dependent on their sequence complementarity. Hence, a key goal in understanding piRNA pathways is to determine mechanisms that modulate piRNA sequences. Here, we identify a protein-protein interaction between the 3′-to-5′ exoribonuclease Nibbler (Nbr) and Piwi that links Nbr activity with piRNA pathways. We show that there is a delicate balance in the interplay between Nbr and Hen1, a methyltransferase involved in 2′-O-methylation at the 3′ terminal nucleotides of piRNAs, thus connecting two genes with opposing activities in the biogenesis of piRNA 3′ ends. With age, piRNAs become shorter and fewer in number, which is coupled with the derepression of select TEs. We demonstrate that activities of Nbr and Hen1 inherently contribute to TE silencing and age-dependent profiles of piRNAs. We propose that antagonistic roles of Nbr and Hen1 define a mechanism to modulate piRNA 3′ ends.
Read more
recent posts
May 29th, 2018
Zaijun ET AL.’s ARTICLE PUBLISHED AT eLIFEFebruary 1st, 2016
HUI’s ARTICLE PUBLISHED AT DEVELOPMENT